Q.:- Calculate the molecular masses of H2, O2, Cl2, CO2, CH4, C2H6, C2H4, NH3, CH3OH.
- Favourite answer Amonia has the formula NH3. Go to the periodic table. Nitrogen has a mass (weight) of 14.
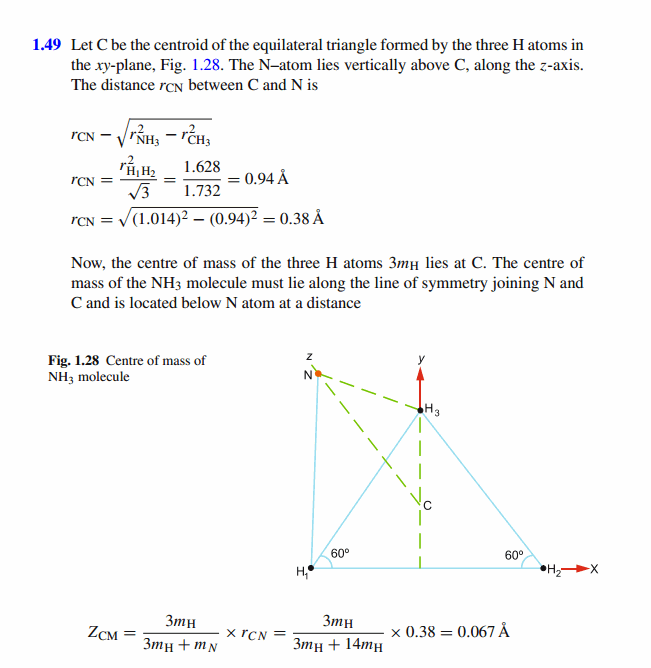
- The nitrogen (N) atom is at the apex of a pyramid, with the three hydrogen atoms forming the base. The nitrogen-to-hydrogen atomic mass ratio is 13.9, and the nitrogen-to-hydrogen distance is L = 10.14 ✕ 10^−11 m. (a) What is the x coordinate of the molecule's center of mass? (b) What is the y coordinate of the molecule's center of mass?
- Atomic Mass # of Atoms: Mass Percent: Hydrogen: H: 1.00794: 5: 26.399%: Nitrogen: N: 14.0067: 1: 84.111% ››.
Massa molar of NH3 is 17.03052 ± 0.00041 g/mol. Mass percent composition, atomic percent compositions and allows to convert from weight to number of moles.
Answer:-
Molecular mass of H2 = 2 X atomic mass of H = 2 X 1u = 2u
Atomic Mass Of Ammonia
Molecular mass of O2 = 2 Xatomic mass of O = 2 X 16u = 32u
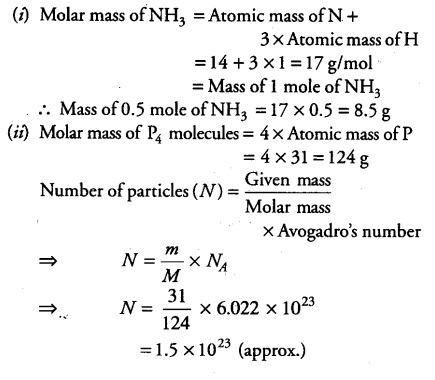
Molecular mass of Cl2 = 2 X atomic mass of Cl = 2 X 35.5u = 71u
Molecular mass of CO2 = atomic mass of C + 2 X atomic mass of O
= 12+ (2+16) = (12 + 32)u = 44u
Molecular mass of CH4 = atomic mass of C+ 4 X atomic mass of H
= 12+ (4X1)u = (12 + 4)u = 16u Palo alto virtual appliance trial.
Molecular mass of C2H6 = 2 X atomic mass of C + 6 X atomic mass of H
= (2 X 12 + 6 X 1)u = (24 + 6)u = 30u
Molecular mass of C2H4 = 2 X atomic mass of C+ 4 X atomic mass of H

= (2 X 12 + 4 X 1)u = (24 + 4)u = 28u
Molecular mass of NH3 = atomic mass of N + 3 X atomic mass of H
Atomic Mass Of Nh4clo4
= (14 + 3 X 1)u = (14 + 3)u = 17u
Molecular mass of CH3OH = atomic mass of C + 3 x atomic mass of H + atomic mass of O
+ atomic mass of H
= (12 + 3 X 1 + 16 + 1)u = (12 + 3 + 17)u = 32u
Weight Of Nh3
